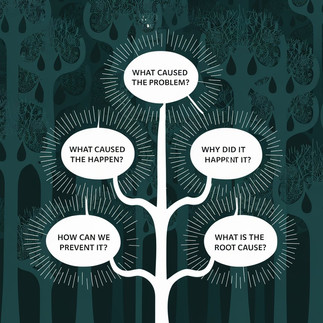
Utilizing the 5 Whys methodology is an effective problem-solving approach that involves asking "why" repeatedly (usually five times) to pinpoint the fundamental cause of an issue. This strategy proves particularly beneficial in the pharmaceutical sector, where strict compliance with regulations, quality benchmarks, and safety measures is paramount.
Example of 5 Whys Analysis in the Pharmaceutical Industry
Case: Out of Specification (OOS) Result for a Stability Study
Scenario: A pharmaceutical company conducts a stability study on a drug product, and the results show that the potency of the drug has fallen below the acceptable limit at the end of the testing period.
Step 1: Identify the Problem
Problem: The potency of the drug product is below specifications during the stability study.
Root Cause Analysis, (RCA)
Step 2: Conduct the 5 Whys Analysis
Why did the potency fail the stability test?
Answer: The active ingredient in the formulation degraded beyond acceptable limits.
Why did the active ingredient degrade beyond acceptable limits?
Answer: The stability samples were stored at an incorrect temperature.
Why were the stability samples stored at an incorrect temperature?
Answer: The stability chamber was malfunctioning and did not maintain the required temperature.
Why was the stability chamber malfunctioning?
Answer: The temperature monitoring system within the chamber failed to alert personnel of the temperature deviation.
Why did the temperature monitoring system fail to alert personnel?
Answer: There was a lack of routine calibration and maintenance checks for the temperature monitoring system.
Root Cause Identified
The root cause of the OOS result was the lack of routine maintenance and calibration for the temperature monitoring system of the stability chamber, leading to improper storage conditions for the stability samples.
Importance of Using the 5 Whys in This Case
Identifying Systemic Issues: This method reveals that the failure is not just a one-off incident but part of a broader maintenance issue.
Preventive Actions: Corrective actions can include establishing a regular maintenance schedule for stability chambers and monitoring systems, which is crucial for compliance with regulatory standards.
Regulatory Compliance: Properly functioning stability chambers are essential for meeting regulatory requirements, ensuring product safety and efficacy.
Quality Improvement: Implementing changes based on root cause analysis fosters a culture of continuous improvement, which is critical in the pharmaceutical sector.
Cost Savings: By addressing the root cause effectively, the company can prevent costly product recalls, regulatory fines, and damage to its reputation.

Full-Length Articles for Better Understanding
1. Understanding Stability Studies in Pharmaceuticals
This article covers the importance of stability studies in drug development, outlining the methodologies, regulatory requirements, and best practices for conducting stability studies effectively.
2. Root Cause Analysis Techniques in the Pharmaceutical Industry
A comprehensive look at various root cause analysis techniques, including the 5 Whys method. This article explains how to implement these techniques and their significance in addressing quality issues.
3. Quality Management Systems in Pharmaceuticals
An in-depth exploration of quality management systems (QMS) in the pharmaceutical industry, emphasizing how proper QMS can prevent deviations and improve overall product quality.
4. Regulatory Compliance in Pharmaceutical Manufacturing
This article discusses the critical aspects of regulatory compliance in pharmaceutical manufacturing, including the role of stability studies and the implications of Out of Specification results.
5. The Role of Preventive Maintenance in Pharmaceutical Equipment
A focused article on the importance of preventive maintenance for equipment in the pharmaceutical industry, detailing best practices and case studies on how maintenance impacts product quality and compliance.
Conclusion
The 5 Whys analysis is an essential tool for identifying the root causes of issues in the pharmaceutical industry, particularly in the context of stability studies. By thoroughly investigating the reasons behind deviations, companies can implement corrective actions that enhance product quality and ensure regulatory compliance. For a deeper understanding of the subject, the suggested articles provide valuable insights into stability studies, root cause analysis, quality management, and compliance in the pharmaceutical sector



I started doing weight training seriously and decided to use steroids to gain mass, I was advised to try SP Laboratories products. After some research I ordered the products through the sp laboratories steroids website. The delivery was fast and the products arrived in good condition with the necessary quality certificates. After just a few weeks of using the steroids I noticed a clear increase in strength and endurance.